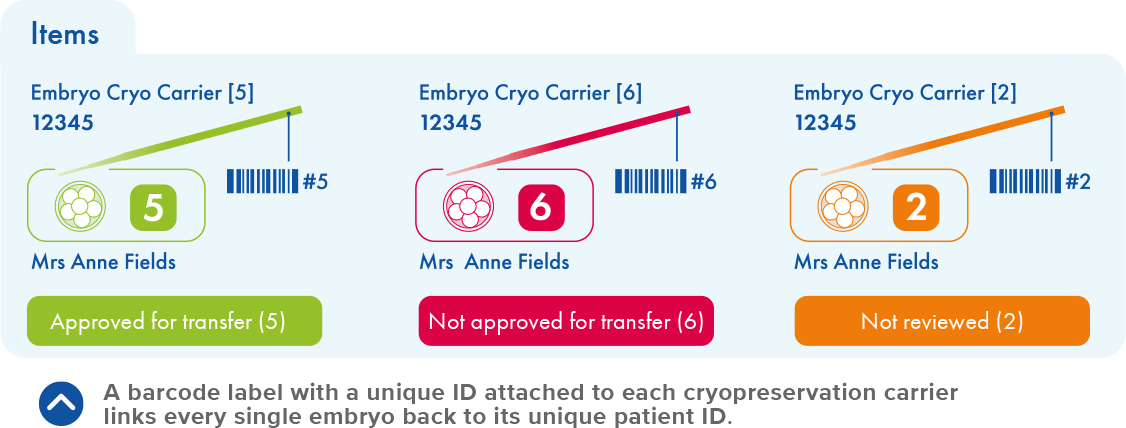
Security Safeguarding
Mis Match Prevention
How RI Witness safeguards your clinic and your patients
Every workstation, where a patient or sample will be treated, is installed with an RI Witness reader. Radio Frequency ID (RFID) ‘tags’ are fixed to all plasticware involved in the patient cycle. As they are used, these tags are registered with each couple’s unique code to identify their gametes, embryos and samples throughout the cycle. Whenever plasticware is brought onto a workstation, the tags are automatically read, identified and logged.
If two incompatible codes are present on a workstation the system immediately sounds an alert. The embryologist can only continue once a match and an action is confirmed. Personalized ID cards or fingerprint readers also capture the patient interactions at entry and exit points.
Using electronic witnessing to minimize IVF errors
How RI Witness safeguards your clinic and your patients
Every workstation where a patient or sample will be treated is installed with an RI Witness reader. Radio Frequency ID (RFID) ‘tags’ are fixed to all plasticware involved in the patient cycle. As they are used, these tags are registered with each couple’s unique code to identify their gametes, embryos and samples throughout the cycle. Whenever plasticware is brought onto a workstation, the tags are automatically read, identified and logged. If two incompatible codes are present on a workstation, the system immediately sounds an alert. The embryologist can only continue once a match and an action is confirmed. Personalized ID cards or fingerprint readers also capture the patient interactions at entry and exit points
Electronic Witnessing technologies: A user’s persepctive
Dr Claire Moran, Lab Manager, Repromed Ireland, Karen Schnauffer, Lab Director, Reproductive Health Group and Dr Steve Troup, RSC
![]()
Listen to our podcast
How RI Witness can help prevent mismatches
With a product focus, Michael J Tucker, PhD FiBiol HCLD, Scientific Director at Shady Grove Fertility, talks to our Americas Regional Manager for Equipment, Amanda Bostian, about helping prevent gamete and embryo mismatches during IVF. Together they explore the impact of mismatches on IVF laboratories, clinics and IVF patients and how RI Witness can help mitigate the risk.
Why choose RFID?
Simply put, RFID technology confers seamless continual monitoring with no interruptions.
It is a proactive system. Double witnessing, reactive systems and hand-held devices all require your embryologists to remember to stop and check.
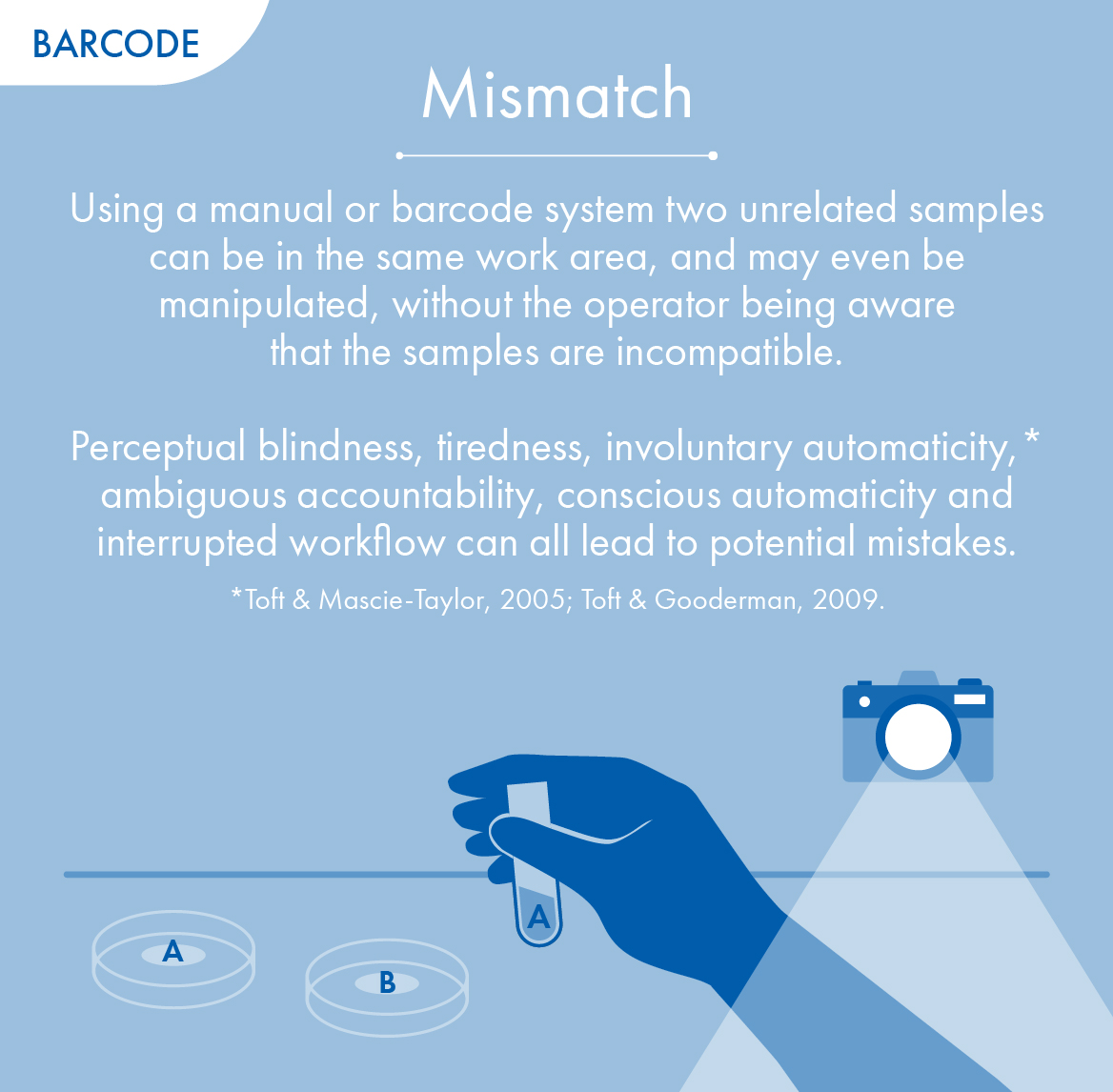
If a single error occurs then the whole process has been compromised – the chain of custody is broken.
Only proactive continual monitoring can prevent two unrelated samples from being in the work area at the same time.
Radio Frequency Identification (RFID) Technology
Self-adhesive RFID tags are attached to all laboratory plasticware
• RFID Readers are situated wherever samples are handled:
– Embryology lab – associated with each stereo microscope
– Andrology – associated with each work area for semen processing
– Reception or egg pick-up and embryo transfer rooms, where patients will be treated
• Each RI Witness work area has a networked tablet or PC:
– User can simply and quickly log into the system using personalized key fob without having to enter a passcode
– Software integrated with your patient database*
• Tags are passive and have no energy source:
– Readers only identify tagged plasticware placed within the work area
RI Witness RFID tags and readers are proven safe for IVF applications and have been used clinically since 2007.¹ More information on RFID safety is available on our website.²
* Subject to compatibility, some programming may be required
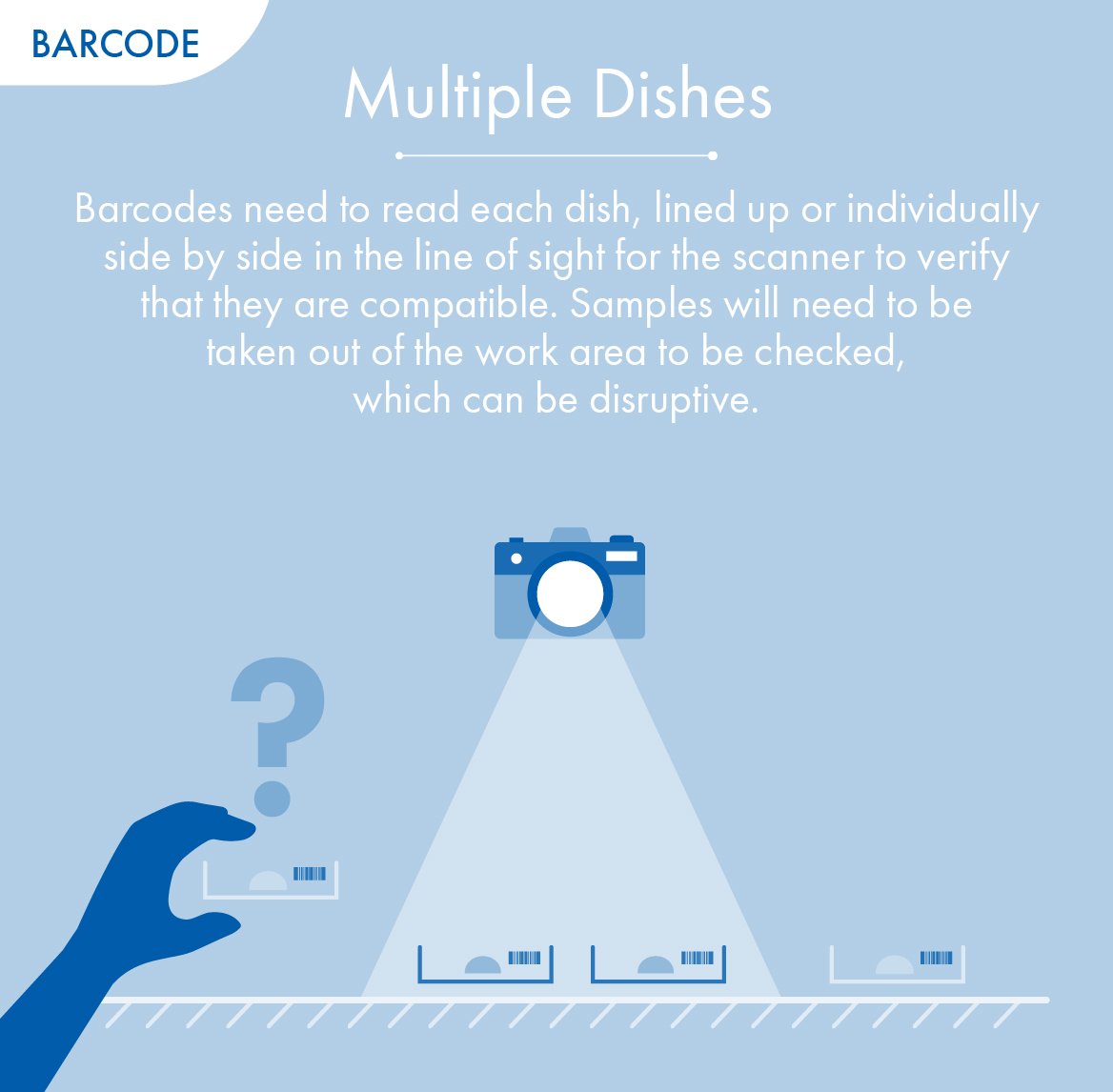
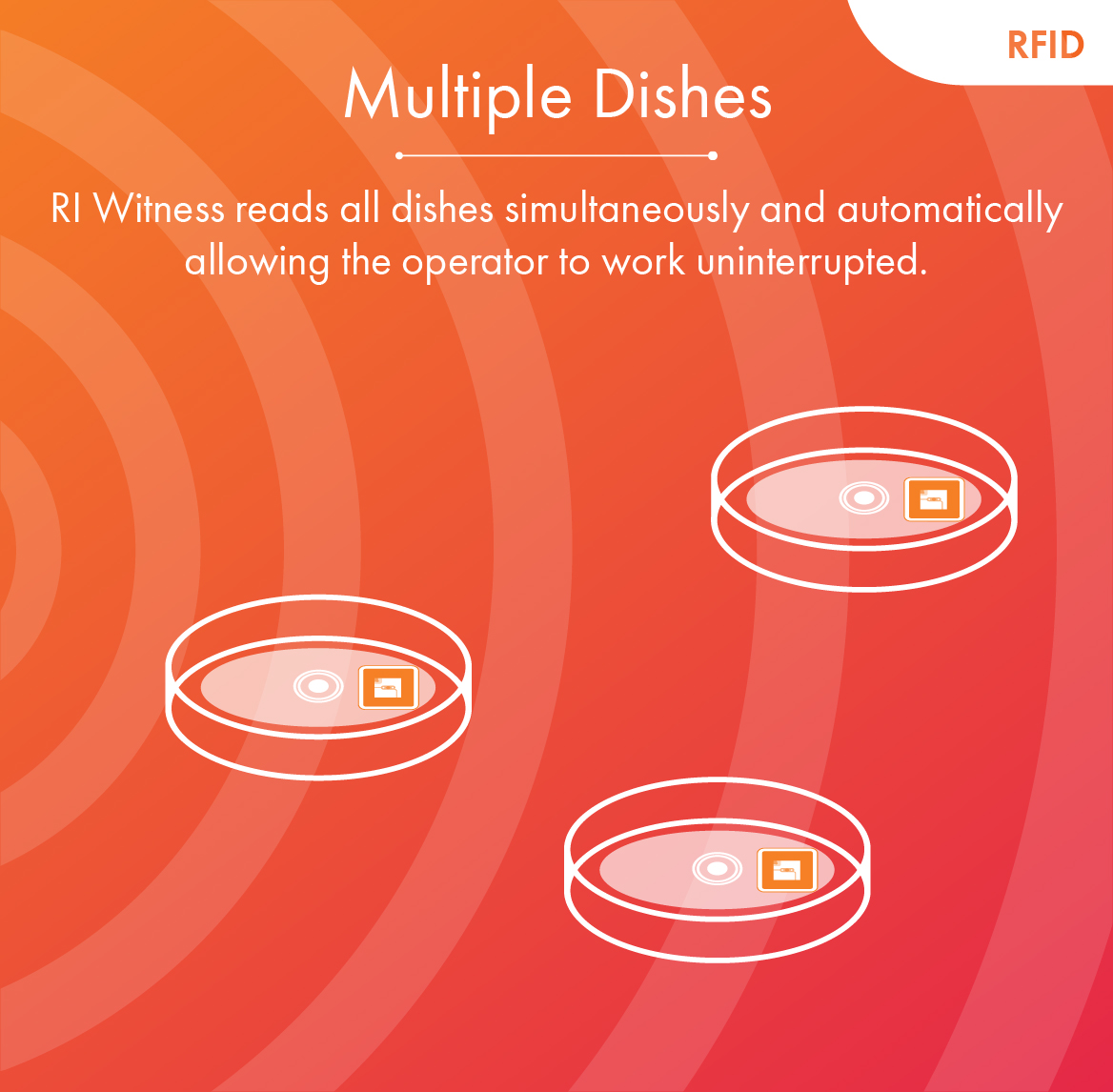
RFID versus Barcodes
RI Witness uses RFID technology instead of barcodes to constantly safeguard the IVF process, from start to finish.
Only RI Witness can monitor every procedure with no workflow interruptions.
Multiple Dishes
Recording Activities


Mismatches

Safety of RFID and RI Witness
We understand it is imperative the conditions in your laboratory are optimized for ART procedures. There is no room for compromise. That is why we use technology that does not harm embryos or humans.
Tested
We asked an independant test centre, the University of Liège, Belgium, to devise a comprehensive experiment to prove out theories about the safe use of RFID with gametes and embryos.
For the test they:
- Increased the output power of the reader by 10x
- Increased the time that the embryos were in the radio frequency field by 70 times what would be expected in an IVF cycle
- In the experiment total exposure was 700x what would be expected in an IVF lab
Results:
- Normal development to mouse blastocyst compared to the control group
- Normal live birth rate (LBR) compared to the control group
- Normal fertility in the female pups with normal 2nd generation LBR
RFID
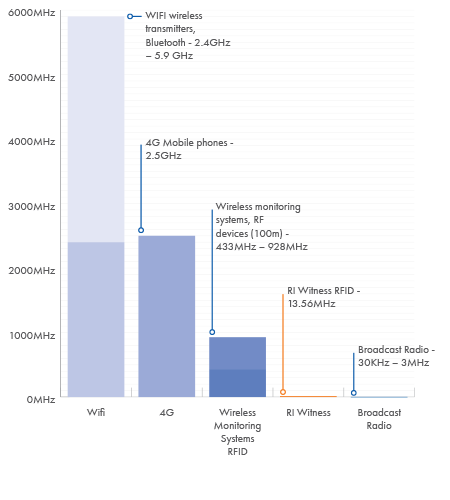
Every day, we are surrounded by radio frequency emissions from modern technology.
The potential effects radio frequencies have on cells and tissues depend on the frequency and power density to which they are exposed.
The facts:
- RI Witness RFID tags do not emit or receive radio waves when they are not on a reader or in the incubator
- The tags are passive, with no internal energy source, so they are only activated when in a 1-5cm range of an RI Witness reader
- Embryos and gametes are only exposed to the radio frequency fields for short time periods
Statistical Evidence
A four-year statistical analysis in one clinic looked at more than 10,000 live birth, before and after the introduction of RI Witness.
The analysis showed no changes to results in both live births and malformations over the two periods7.
Certified
Our embryology heated plates are CE marked as medical devices (GB98/13044) and FDA cleared. They offer highly stable heating across the reader.
Our tags and labels are batch tested to MEA >80% survival to blastocyst stage6.
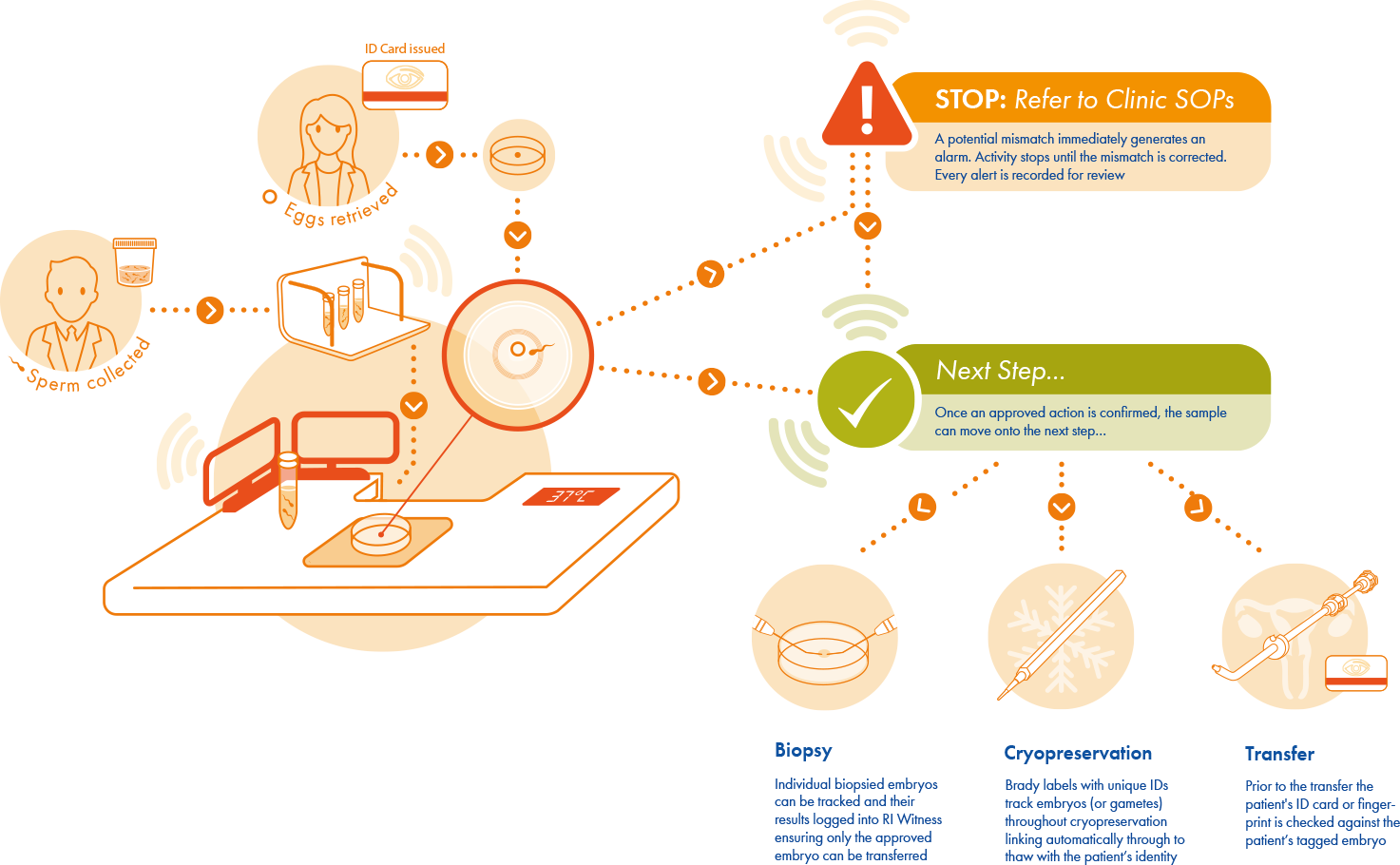
How PGT cycles are managed in RI Witness
Each year, an increasing number of preimplantation genetic testing (PGT) cycles are performed all over the world, which places a greater and more complex burden on the laboratory workload. Therefore, the accommodation of these additional steps in laboratory security protocols is imperative.
With RI Witness, whether biopsying for preimplantation genetic testing (PGT) is carried out, and/or cryopreservation and transfer – your patients are always safeguarded from misidentification.
Every One Is Unique
By generating a unique identity every embryo is visible throughout cryopreservation and transfer, tracked separately yet alongside any others from the same patients.
Product Specifications
| Work Areas | One work area required for each critical working location. Microsoft Windows based PC or Tablet needed for each work area. Readers available heated or unheated. RFID reader frequency: 13.56MHz |
| Barcode Compatibility (Traceability) | Compatible with GS1 barcodes (GS1-128) |
| Barcode Scanner (Traceability) | Compatible with USB (Keyboard wedge) fixed and hand held scanners |
| Camera Compatibility (Imaging) | Research Instruments’ DC1 & DC2, Analogue cameras |
| RI Witness™ Manager (Client Software) PC System Requirements | Operating Systems: Windows 11, Windows 10 |
| Server / Network Requirements | Microsoft SQL Server required (not supplied). Network Point required for each work area |
Order Codes
The Order Codes for RI Witness™ will depend on your particular configuration.
Brochures
RI Witness IQ Solution Brochure -German
RI Witness IQ Solution Brochure – French
RI Witness IQ Solution Brochure – Italian
RI Witness IQ Solution Brochure – Spanish
RI Witness IQ Solution Brochure – Turkish
RI Witness Patient Flyer – US
RI Witness Patient Flyer – Turkish
RI Witness Patient Flyer – Portuguese
RI Witness Patient Flyer – Czech
RI Witness Patient Flyer – Greek
RI Witness Patient Flyer – Spanish
RI Witness Patient Flyer – Dutch
RI Witness Patient Flyer – German
RI Witness Patient Flyer – French
RI Witness Patient Flyer – Italian
RI Witness Patient Flyer – Arabic
RI Witness Patient Flyer – Bulgarian
RI Witness Patient Flyer – Danish
Same Sex Couple RI Witness Patient Flyer
RI Witness Patient Flyer – Same Sex Couple Male
RI Witness Patient Flyer – Same Sex Couple Female
RI Witness Patient Flyer – Same Sex Couple Female – Spanish
RI Witness Patient Flyer – Same Sex Couple Female – Czech
RI Witness Patient Flyer – Same Sex Couple Male – Czech
RI Witness Patient Flyer – Same Sex Couple Female – French
RI Witness Patient Flyer – Same Sex Couple Female – Portuguese
RI Witness Patient Flyer – Same Sex Couple Male – Portuguese
RI Witness Patient Flyer Same Sex Couple Male – Bulgarian
RI Witness Patient Flyer Same Sex Couple Female – Bulgarian

 My Clinic is in the United States
My Clinic is in the United States My Clinic is in Canada
My Clinic is in Canada
 Trusting the transfer process
Trusting the transfer process Thawing and transfer
Thawing and transfer